Empirical Formula: Once the experimental method is observed, the molecular method for a compound may be decided if the molar mass of the compound is known. Simply forecast the mass of the empirical creed and divide the molar mass of the compound via way of means of the mass of the empirical method to locate the ratio between the molecular method and the empirical creed. Multiply all of the atoms (among) via way of means of this ratio to locate the molecular creed. In chemistry, the empirical method of a chemical compound is the only productive integer ratio of atoms present in a compound.
An easy instance of this idea is that the empirical creed of sulfur monoxide, or SO, could truly be SO, as is the experimental formula of disulfur dioxide, S2O2. Thus, sulfur monoxide and disulfur dioxide, each compound of sulphur and oxygen, have an equal empirical method. However, their atomic formulas, which explicit the number of atoms in every molecule of a chemical compound, aren’t equal.
Empirical Formula Calculator
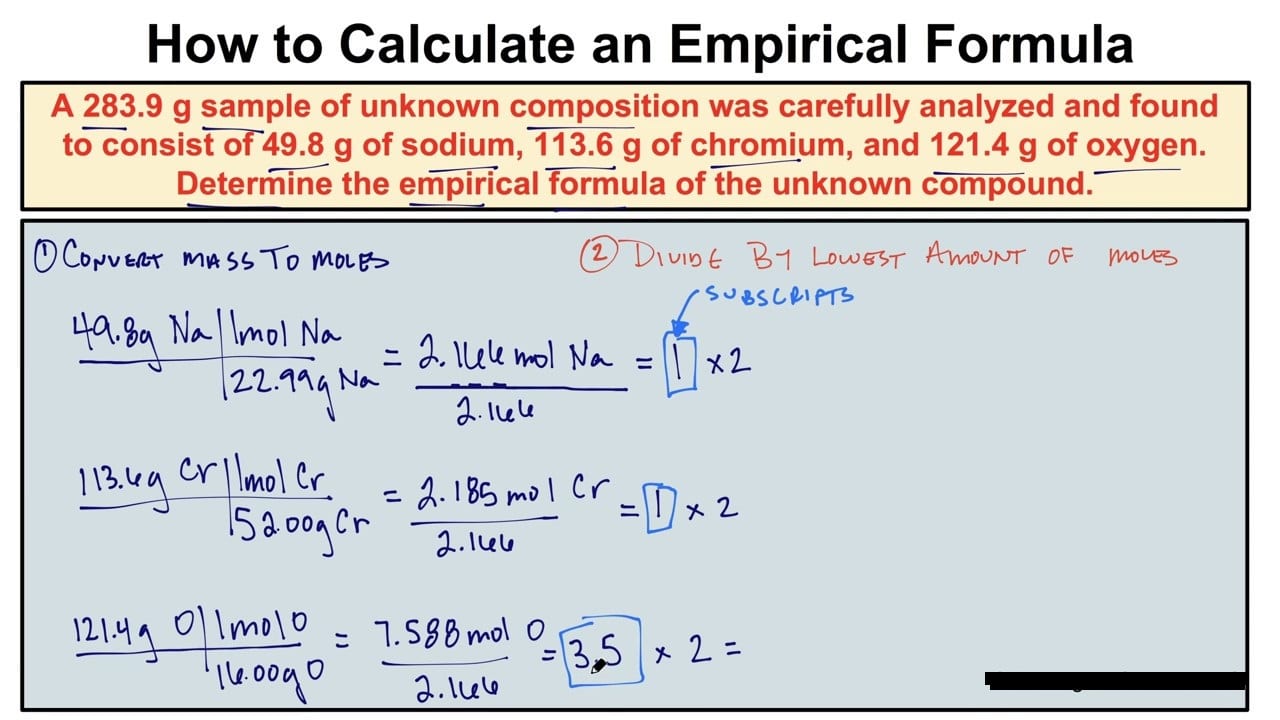
The empirical creed of a compound is the straightforward complete quantity ratio of every kind of atom in a compound. It may be similar to the compound’s molecular creed, however now no longer always. An empirical creed may be calculated from instruction approximately the mass of every detail in a commixture or from the share composition. To calculate the experimental method, you ought to first decide the relative loads of the different factors gift. You can both use mass statistics in grams or percentage composition.
For percentage balance, we expect the whole percentage of a commixture is identical to one hundred% and the percentage formation is equal in grams. For instance, the whole mass of the commixture is one hundred grams. If a commixture contained sixty-eight ribbons, nine% hydrogen, and 23% oxygen, we’d expect sixty-eight grams of carbon, nine grams of hydrogen, and 23 grams of oxygen.
How To Find Empirical Formula
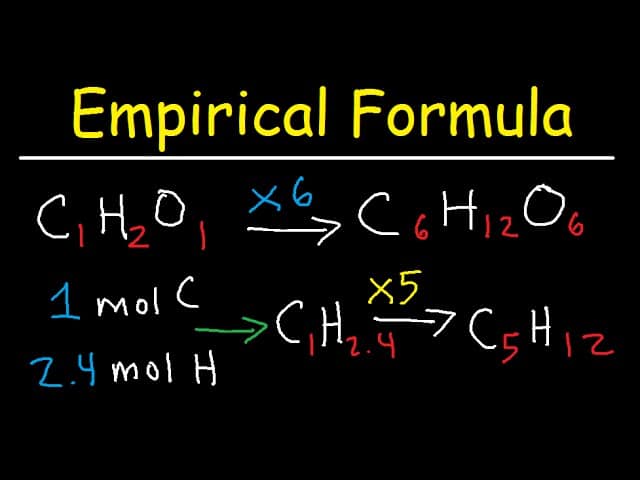
You can use the experimental method to locate the atomic method in case you recognize the molar mass of the compound. To do this, calculate the empirical method mass after which divide the compound molar throng via way of means of the empirical method mass. This offers you the ratio among the atomic and empirical formulas. Multiply all of the subscripts inside the empirical method via way of means of this ratio to get the indexes for the molecular creed. Also Check – Numbers in French – The Ultimate Guide
Here’s the way to locate an empirical method while given percentage composition:
- Assume which you have one hundred g of the unknown compound. The splendor of this little trick is which you simply present yourself with an equal quantity of grams of every elemental thing as its contribution to the percentage composition. For instance, in case you expect which you have one hundred g of a compound composed of 60.3% magnesium and 39.7% oxygen, you already know which you have 60.three g of magnesium and 39.7 g of oxygen. (The simplest time you don’t do that is if the hassle specially offers you loads of every detail gift with inside the unknown compound.)
- Convert the loads from Step 1 into moles the usage of the molar mass.
- Determine which detail has the smallest mole value. Then divide all of the mole values you calculated in Step 2 via way of means of this smallest value. This department yields the mole ratios of the factors of the compound.
- If any of your mole ratios aren’t complete numbers, multiply all numbers via way of means of the smallest viable thing that produces complete-quantity mole ratios for all of the factors. For illustration, when you have 1 nitrogen atom for each 0.five oxygen atoms in a compound, the experimental method isn’t always N1O0.five. Such a creed casually recommends that an oxygen atom has been split, an object that could create a small-scale nuclear explosion. Though impressive-sounding, this scheme is nearly truly false. Far more reasonable is that the atoms of nitrogen and oxygen are incorporating in a 1: 0.five ratios however accomplish that in a bigger however comparable ratio of 2: 1. The empirical method is consequently N2O.Because the authentic percentage composition statistics are normally experimental, count on to look a chunk of blunders inside the numbers. For instance, 2.03 might be inside experimental blunders of 2, 2. ninety-nine might be three, and so on.
- Write the empirical method via way of means of attaching those complete-quantity mole ratios as subscripts to the chemical image of every detail.
Order the factors in step with the overall policies for naming ionic and molecular compounds.
Empirical Vs Molecular Formula
The empirical method of a compound is described as the method that suggests the ratio of factors gift with inside the compound, however now no longer the real numbers of atoms observed inside the molecule. The ratios are denoted via way of means of subscripts after the detail symbols.
Also Known As The empirical method is likewise referred to as the only method due to the fact the subscripts are the smallest complete numbers that suggest the ratio of factors. Related – Diatomic Elements | Definition, Example & More